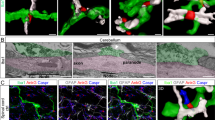
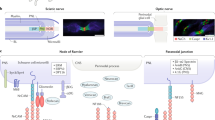
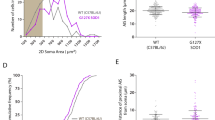
Abstract
Saltatory conduction of action potentials along myelinated axons depends on the nodes of Ranvier — small unmyelinated axonal domains where voltage-gated sodium channels are concentrated. Our knowledge of the complex molecular composition of these axonal domains continues to accumulate, although the mechanisms of nodal assembly, which have been elucidated in the PNS, remain only partially understood in the CNS. Besides the key role of the nodes in accelerating conduction, nodal variations are thought to allow the fine tuning of axonal conduction speed to meet information processing needs. In addition, through their multiple glial contacts, nodes seem to be important for neuron–glia interactions. As we highlight in this Review, the disorganization of axonal domains has been implicated in the pathophysiology of various neurological diseases. In multiple sclerosis, for example, nodal and perinodal disruption following demyelination, with subsequent changes in ion channel distribution, leads to altered axonal conduction and integrity. The nodal clusters regenerate concurrently with but also prior to remyelination, allowing the restoration of axonal conduction. In this article, we review current knowledge of the organization and function of nodes of Ranvier in the CNS. We go on to discuss dynamic changes in the nodes during demyelination and remyelination, highlighting the impact of these changes on neuronal physiology in health and disease as well as the associated therapeutic implications.
Key points
-
Nodes of Ranvier allow high-speed saltatory propagation of action potentials along myelinated fibres.
-
A range of mechanisms contribute to nodal protein clustering in the CNS.
-
The presence of multiple glial contacts at the nodal surface suggests that the CNS node might act as an axoglial hub, opening up new perspectives regarding neuroglial communication.
-
Variations in node of Ranvier length and diameter allow the fine-tuning of axonal conduction speed to meet information processing needs at the network scale.
-
Nodal domains might represent sites of axonal vulnerability in demyelinating diseases.
-
The disruption of node of Ranvier and paranodal junctions in CNS demyelinating diseases is associated with voltage-gated sodium and potassium channel redistribution, affecting action potential propagation and axonal integrity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Poliak, S. & Peles, E. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4, 968–980 (2003).
Faivre-Sarrailh, C. & Devaux, J. J. Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front. Cell. Neurosci. 7, 196 (2013).
Rasband, M. N. & Peles, E. The nodes of Ranvier: molecular assembly and maintenance. Cold Spring Harb. Perspect. Biol. 8, a020495 (2015).
Salzer, J. L., Brophy, P. J. & Peles, E. Molecular domains of myelinated axons in the peripheral nervous system. Glia 56, 1532–1540 (2008).
Brohawn, S. G. et al. The mechanosensitive ion channel TRAAK is localized to the mammalian node of Ranvier. eLife 8, e50403 (2019).
Kanda, H. et al. TREK-1 and TRAAK are principal K+ channels at the nodes of Ranvier for rapid action potential conduction on mammalian myelinated afferent nerves. Neuron 104, 960–971 (2019).
Seidl, A. H. Regulation of conduction time along axons. Neuroscience 276, 126–134 (2014).
Arancibia-Carcamo, I. L. & Attwell, D. The node of Ranvier in CNS pathology. Acta Neuropathol. 128, 161–175 (2014).
Ranvier, L. Contribution à l’histologie et à la physiologie des nerfs périphériques [French]. C. R. Acad. Sci. 73, 1168–1171 (1871).
Ranvier, L-A. Leçons sur l’Histologie du Système Nerveux, par M. L. Ranvier, recueillies par M. Ed. Weber (Librairie F. Savy, 1878).
Lillie, R. S. Factors affecting transmission and recovery in the passive iron nerve model. J. Gen. Physiol. 7, 473–507 (1925).
Peters, A. The formation and structure of myelin sheaths in the central nervous system. J. Biophys. Biochem. Cytol. 8, 431–446 (1960).
Uzman, B. G. & Villegas, G. M. A comparison of nodes of Ranvier in sciatic nerves with node-like structures in optic nerves of the mouse. J. Biophys. Biochem. Cytol. 7, 761–762 (1960).
Metuzals, J. Ultrastructure of myelinated nerve fibers in the central nervous system of the frog. J. Ultrastruct. Res. 8, 30–47 (1963)
Salzer, J. L. Polarized domains of myelinated axons. Neuron 40, 297–318 (2003).
Arancibia-Carcamo, I. L. et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife 6, e23329 (2017).
Micheva, K. D. et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife 5, e15784 (2016).
Micheva, K. D. et al. Distinctive structural and molecular features of myelinated inhibitory axons in human neocortex. eNeuro 5, ENEURO.0297-18.2018 (2018).
Landon, D. N. & Williams, P. L. Ultrastructure of the node of Ranvier. Nature 199, 575–577 (1963).
Pannese, E. Neurocytology: Fine Structure of Neurons, Nerve Processes, and Neuroglial Cells (Springer International Publishing, 2015).
Ford, M. C. et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun. 6, 8073–8073 (2015).
Rosenbluth, J. Electrophysiology and morphology of myelinated nerve fibers. V. Intramembranous particle distribution in nerve fiber membranes. Experientia 39, 953–963 (1983).
Edgar, J. M., McCulloch, M. C., Thomson, C. E. & Griffiths, I. R. Distribution of mitochondria along small-diameter myelinated central nervous system axons. J. Neurosci. Res. 86, 2250–2257 (2008).
Ohno, N. et al. Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J. Neurosci. 31, 7249–7258 (2011).
D’Este, E. et al. Subcortical cytoskeleton periodicity throughout the nervous system. Sci. Rep. 6, 22741 (2016).
D’Este, E., Kamin, D., Balzarotti, F. & Hell, S. W. Ultrastructural anatomy of nodes of Ranvier in the peripheral nervous system as revealed by STED microscopy. Proc. Natl Acad. Sci. USA 114, E191–E199 (2017).
Black, J. A. & Waxman, S. G. The perinodal astrocyte. Glia 1, 169–183 (1988).
Butt, A. M., Duncan, A. & Berry, M. Astrocyte associations with nodes of Ranvier: ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J. Neurocytol. 23, 486–499 (1994).
Butt, A. M. et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 26, 84–91 (1999).
Serwanski, D. R., Jukkola, P. & Nishiyama, A. Heterogeneity of astrocyte and NG2 cell insertion at the node of Ranvier. J. Comp. Neurol. 525, 535–552 (2017).
Ffrench-Constant, C., Miller, R. H., Kruse, J., Schachner, M. & Raff, M. C. Molecular specialization of astrocyte processes at nodes of Ranvier in rat optic nerve. J. Cell Biol. 102, 844–852 (1986).
Dutta, D. J. et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl Acad. Sci. USA 115, 11832–11837 (2018).
Hildebrand, C. & Waxman, S. G. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. J. Comp. Neurol. 224, 25–37 (1984).
De Biase, L. M., Pucak, M. L., Kang, S. H., Rodriguez, S. N. & Bergles, D. E. Sparse interaction between oligodendrocyte precursor cells (NG2+ cells) and nodes of Ranvier in the central nervous system. bioRxiv https://doi.org/10.1101/185801 (2017).
Zhang, J., Yang, X., Zhou, Y., Fox, H. & Xiong, H. Direct contacts of microglia on myelin sheath and Ranvier’s node in the corpus callosum in rats. J. Biomed. Res. 33, 192–200 (2019).
Oohashi, T., Edamatsu, M., Bekku, Y. & Carulli, D. The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp. Neurol. 274, 134–144 (2015).
Dermietzel, R. Junctions in the central nervous system of the cat. II. A contribution to the tertiary structure of the axonal-glial junctions in the paranodal region of the node of Ranvier. Cell Tissue Res. 148, 577–586 (1974).
Rosenbluth, J., Petzold, C. & Peles, E. Dependence of paranodal junctional gap width on transverse bands. J. Comp. Neurol. 520, 2774–2784 (2012).
Waxman, S. G. & Ritchie, J. M. Molecular dissection of the myelinated axon. Ann. Neurol. 33, 121–136 (1993).
Catterall, W. A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26, 13–25 (2000).
Patton, D. E., Isom, L. L., Catterall, W. A. & Goldin, A. L. The adult rat brain beta 1 subunit modifies activation and inactivation gating of multiple sodium channel alpha subunits. J. Biol. Chem. 269, 17649–17655 (1994).
Isom, L. L. et al. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83, 433–442 (1995).
Chen, C. et al. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proc. Natl Acad. Sci. USA 99, 17072 (2002).
Namadurai, S. et al. A new look at sodium channel β subunits. Open Biol. 5, 140192 (2015).
Lopez-Santiago, L. F. et al. Sodium channel beta 2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J. Neurosci. 26, 7984–7994 (2006).
Duflocq, A., Le Bras, B., Bullier, E., Couraud, F. & Davenne, M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol. Cell. Neurosci. 39, 180–192 (2008).
Van Wart, A. & Matthews, G. Impaired firing and cell-specific compensation in neurons lacking nav1.6 sodium channels. J. Neurosci. 26, 7172–7180 (2006).
Ogiwara, I. et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 27, 5903–5914 (2007).
Lorincz, A. & Nusser, Z. Cell-type-dependent molecular composition of the axon initial segment. J. Neurosci. 28, 14329–14340 (2008).
Tian, C., Wang, K., Ke, W., Guo, H. & Shu, Y. Molecular identity of axonal sodium channels in human cortical pyramidal cells. Front. Cell. Neurosci. 8, 297–297 (2014).
Van Wart, A., Trimmer, J. S. & Matthews, G. Polarized distribution of ion channels within microdomains of the axon initial segment. J. Comp. Neurol. 500, 339–352 (2007).
Schaller, K. L. & Caldwell, J. H. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum 2, 2–9 (2003).
Caldwell, J. H., Schaller, K. L., Lasher, R. S., Peles, E. & Levinson, S. R. Sodium channel Na(v)1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc. Natl Acad. Sci. USA 97, 5616–5620 (2000).
Tzoumaka, E. et al. Differential distribution of the tetrodotoxin-sensitive rPN4/NaCh6/Scn8a sodium channel in the nervous system. J. Neurosci. Res. 60, 37–44 (2000).
Schafer, D. P., Custer, A. W., Shrager, P. & Rasband, M. N. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol. 2, 69–79 (2006).
Boiko, T. et al. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30, 91–104 (2001).
Zhou, W. & Goldin, A. L. Use-dependent potentiation of the Nav1.6 sodium channel. Biophys. J. 87, 3862–3872 (2004).
Herzog, R. I., Liu, C., Waxman, S. G. & Cummins, T. R. Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties. J. Neurosci. 23, 8261–8270 (2003).
Arroyo, E. J. et al. Genetic dysmyelination alters the molecular architecture of the nodal region. J. Neurosci. 22, 1726–1737 (2002).
Kazen-Gillespie, K. A. et al. Cloning, localization, and functional expression of sodium channel beta1A subunits. J. Biol. Chem. 275, 1079–1088 (2000).
Buffington, S. A. & Rasband, M. N. Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. J. Neurosci. 33, 6191–6202 (2013).
Chen, C. et al. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J. Neurosci. 24, 4030–4042 (2004).
Ratcliffe, C. F., Westenbroek, R. E., Curtis, R. & Catterall, W. A. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154, 427–434 (2001).
Kaplan, M. R. et al. Differential control of clustering of the sodium channels Nav1.2 and Nav1.6 at developing CNS nodes of Ranvier. Neuron 30, 105–119 (2001).
Srinivasan, J., Schachner, M. & Catterall, W. A. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl Acad. Sci. USA 95, 15753 (1998).
Patino, G. A. & Isom, L. L. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease. Neurosci. Lett. 486, 53–59 (2010).
Malhotra, J. D. et al. Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. J. Biol. Chem. 277, 26681–26688 (2002).
Kim, D. Y. et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat. Cell Biol. 9, 755–764 (2007).
Devaux, J. J., Kleopa, K. A., Cooper, E. C. & Scherer, S. S. KCNQ2 is a nodal K+ channel. J. Neurosci. 24, 1236–1244 (2004).
Pan, Z. et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 26, 2599–2613 (2006).
Schwarz, J. R. et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J. Physiol. 573, 17–34 (2006).
Battefeld, A., Tran, B. T., Gavrilis, J., Cooper, E. C. & Kole, M. H. P. Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J. Neurosci. 34, 3719–3732 (2014).
Devaux, J. et al. Kv3.1b is a novel component of CNS nodes. J. Neurosci. 23, 4509–4518 (2003).
Lien, C.-C. & Jonas, P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J. Neurosci. 23, 2058–2068 (2003).
Davis, J. Q., Lambert, S. & Bennett, V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J. Cell Biol. 135, 1355–1367 (1996).
Zhang, A. et al. Neurofascin 140 is an embryonic neuronal neurofascin isoform that promotes the assembly of the node of Ranvier. J. Neurosci. 35, 2246–2254 (2015).
Grumet, M., Mauro, V., Burgoon, M. P., Edelman, G. M. & Cunningham, B. A. Structure of a new nervous system glycoprotein, Nr-CAM, and its relationship to subgroups of neural cell adhesion molecules. J. Cell Biol. 113, 1399–1412 (1991).
Custer, A. W. et al. The role of the ankyrin-binding protein NrCAM in node of Ranvier formation. J. Neurosci. 23, 10032–10039 (2003).
Feinberg, K. et al. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron 65, 490–502 (2010).
Rios, J. C. et al. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J. Neurosci. 20, 8354–8364 (2000).
Lustig, M. et al. Nr-CAM and neurofascin interactions regulate ankyrin G and sodium channel clustering at the node of Ranvier. Curr. Biol. 11, 1864–1869 (2001).
Labasque, M. & Faivre-Sarrailh, C. GPI-anchored proteins at the node of Ranvier. FEBS Lett. 584, 1787–1792 (2010).
Leterrier, C., Brachet, A., Dargent, B. & Vacher, H. Determinants of voltage-gated sodium channel clustering in neurons. Semin. Cell Dev. Biol. 22, 171–177 (2011).
Berghs, S. et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J. Cell Biol. 151, 985–1002 (2000).
Jenkins, S. M. & Bennett, V. Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc. Natl Acad. Sci. USA 99, 2303–2308 (2002).
Garrido, J. J. et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300, 2091–2094 (2003).
Gasser, A. et al. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J. Neurosci. 32, 7232–7243 (2012).
Bekku, Y. & Oohashi, T. Neurocan contributes to the molecular heterogeneity of the perinodal ECM. Arch. Histol. Cytol. 73, 95–102 (2010).
Bekku, Y. et al. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J. Neurosci. 30, 3113–3123 (2010).
Bekku, Y., Rauch, U., Ninomiya, Y. & Oohashi, T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J. Neurochem. 108, 1266–1276 (2009).
Dours-Zimmermann, M. T. et al. Versican V2 assembles the extracellular matrix surrounding the nodes of Ranvier in the CNS. J. Neurosci. 29, 7731–7742 (2009).
Hedstrom, K. L. et al. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 178, 875–886 (2007).
Susuki, K. et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron 78, 469–482 (2013).
Zimmermann, D. R. & Dours-Zimmermann, M. T. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 130, 635–653 (2008).
Melendez-Vasquez, C. et al. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia 52, 301–308 (2005).
Weber, P. et al. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J. Neurosci. 19, 4245–4262 (1999).
Bartsch, U., Bartsch, S., Dörries, U. & Schachner, M. Immunohistological localization of tenascin in the developing and lesioned adult mouse optic nerve. Eur. J. Neurosci. 4, 338–352 (1992).
Fawcett, J. W., Oohashi, T. & Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 20, 451–465 (2019).
Susuki, K. & Rasband, M. N. Molecular mechanisms of node of Ranvier formation. Curr. Opin. Cell Biol. 20, 616–623 (2008).
Charles, P. et al. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr. Biol. 12, 217–220 (2002).
Girault, J.-A. & Peles, E. Development of nodes of Ranvier. Curr. Opin. Neurobiol. 12, 476–485 (2002).
Bhat, M. A. et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30, 369–383 (2001).
Boyle, M. E. et al. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30, 385–397 (2001).
Collinson, J. M., Marshall, D., Gillespie, C. S. & Brophy, P. J. Transient expression of neurofascin by oligodendrocytes at the onset of myelinogenesis: implications for mechanisms of axon-glial interaction. Glia 23, 11–23 (1998).
Einheber, S. et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 139, 1495–1506 (1997).
Menegoz, M. et al. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron 19, 319–331 (1997).
Sherman, D. L. et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 48, 737–742 (2005).
Tait, S. et al. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J. Cell Biol. 150, 657–666 (2000).
Ogawa, Y. et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J. Neurosci. 26, 5230–5239 (2006).
Chang, K.-J. et al. Glial ankyrins facilitate paranodal axoglial junction assembly. Nat. Neurosci. 17, 1673–1681 (2014).
Rasband, M. N. Clustered K+ channel complexes in axons. Neurosci. Lett. 486, 101–106 (2010).
Wang, H., Kunkel, D. D., Martin, T. M., Schwartzkroin, P. A. & Tempel, B. L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365, 75–79 (1993).
Vabnick, I. & Shrager, P. Ion channel redistribution and function during development of the myelinated axon. J. Neurobiol. 37, 80–96 (1998).
Traka, M. et al. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol. 162, 1161–1172 (2003).
Eshed-Eisenbach, Y. & Peles, E. The clustering of voltage-gated sodium channels in various excitable membranes. Dev. Neurobiol. https://doi.org/10.1002/dneu.22728 (2019).
Ghosh, A., Sherman, D. L. & Brophy, P. J. The axonal cytoskeleton and the assembly of nodes of Ranvier. Neuroscientist 24, 104–110 (2018).
Rasband, M. N. et al. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 19, 7516–7528 (1999).
Eshed-Eisenbach, Y. & Peles, E. The making of a node: a co-production of neurons and glia. Curr. Opin. Neurobiol. 23, 1049–1056 (2013).
Susuki, K., Otani, Y. & Rasband, M. N. Submembranous cytoskeletons stabilize nodes of Ranvier. Exp. Neurol. 283, 446–451 (2016).
Horresh, I., Bar, V., Kissil, J. L. & Peles, E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J. Neurosci. 30, 2480–2489 (2010).
Zhang, C., Susuki, K., Zollinger, D. R., Dupree, J. L. & Rasband, M. N. Membrane domain organization of myelinated axons requires βII spectrin. J. Cell Biol. 203, 437–443 (2013).
Brivio, V., Faivre-Sarrailh, C., Peles, E., Sherman, D. L. & Brophy, P. J. Assembly of CNS nodes of Ranvier in myelinated nerves is promoted by the axon cytoskeleton. Curr. Biol. 27, 1068–1073 (2017).
Zonta, B. et al. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J. Cell Biol. 181, 1169–1177 (2008).
Pillai, A. M. et al. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J. Neurosci. Res. 87, 1773–1793 (2009).
Rios, J. C. et al. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J. Neurosci. 23, 7001–7011 (2003).
Kaplan, M. R. et al. Induction of sodium channel clustering by oligodendrocytes. Nature 386, 724–728 (1997).
Freeman, S. A. et al. Acceleration of conduction velocity linked to clustering of nodal components precedes myelination. Proc. Natl Acad. Sci. USA 112, E321–E328 (2015).
Dubessy, A.-L. et al. Role of a Contactin multi-molecular complex secreted by oligodendrocytes in nodal protein clustering in the CNS. Glia 67, 2248–2263 (2019).
Thetiot, M. et al. An alternative mechanism of early nodal clustering and myelination onset in GABAergic neurons of the central nervous system. Glia https://doi.org/10.1002/glia.23812 (2020).
Yang, Y., Ogawa, Y., Hedstrom, K. L. & Rasband, M. N. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J. Cell Biol. 176, 509–519 (2007).
Hill, A. S. et al. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 4, e1000317 (2008).
Yang, Y., Lacas-Gervais, S., Morest, D. K., Solimena, M. & Rasband, M. N. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J. Neurosci. 24, 7230–7240 (2004).
Dzhashiashvili, Y. et al. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 177, 857–870 (2007).
Ho, T. S.-Y. et al. A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat. Neurosci. 17, 1664–1672 (2014).
Ratcliffe, C. F. et al. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase beta. Nat. Neurosci. 3, 437–444 (2000).
Xiao, Z. C. et al. Tenascin-R is a functional modulator of sodium channel beta subunits. J. Biol. Chem. 274, 26511–26517 (1999).
Kazarinova-Noyes, K. et al. Contactin associates with Na+ channels and increases their functional expression. J. Neurosci. 21, 7517–7525 (2001).
Waxman, S. G. & Foster, R. E. Development of the axon membrane during differentiation of myelinated fibres in spinal nerve roots. Proc. R. Soc. Lond. B Biol. Sci. 209, 441–446 (1980).
Waxman, S. G., Black, J. A. & Foster, R. E. Freeze-fracture heterogeneity of the axolemma of premyelinated fibers in the CNS. Neurology 32, 418–421 (1982).
Bonetto, G. et al. Selective axonal expression of the Kv1 channel complex in pre-myelinated GABAergic hippocampal neurons. Front. Cell. Neurosci. 13, 222 (2019).
Çolakoğlu, G., Bergstrom-Tyrberg, U., Berglund, E. O. & Ranscht, B. Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc. Natl Acad. Sci. USA 111, E394–E403 (2014).
Brümmendorf, T. et al. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron 10, 711–727 (1993).
Peles, E. et al. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell 82, 251–260 (1995).
Rathjen, F. G., Wolff, J. M. & Chiquet-Ehrismann, R. Restrictin: a chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development 113, 151–164 (1991).
Zacharias, U., Nörenberg, U. & Rathjen, F. G. Functional interactions of the immunoglobulin superfamily member F11 are differentially regulated by the extracellular matrix proteins tenascin-R and tenascin-C. J. Biol. Chem. 274, 24357–24365 (1999).
Black, J. A., Foster, R. E. & Waxman, S. G. Rat optic nerve: freeze-fracture studies during development of myelinated axons. Brain Res. 250, 1–20 (1982).
Jinno, S. et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 27, 8790–8804 (2007).
Bonifazi, P. et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424 (2009).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Huxley, A. F. & Stämpfli, R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. 108, 315–339 (1949).
Tasaki, I. New measurements of the capacity and the resistance of the myelin sheath and the nodal membrane of the isolated frog nerve fiber. Am. J. Physiol. 181, 639–650 (1955).
Cohen, C. C. H. et al. Saltatory conduction along myelinated axons involves a periaxonal nanocircuit. Cell 180, 311–322.e15 (2020).
Rushton, W. A. H. A theory of the effects of fibre size in medullated nerve. J. Physiol. 115, 101–122 (1951).
Waxman, S. G. & Bennett, M. V. Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat. New Biol. 238, 217–219 (1972).
Debanne, D., Campanac, E., Bialowas, A., Carlier, E. & Alcaraz, G. Axon physiology. Physiol. Rev. 91, 555–602 (2011).
Freeman, S. A., Desmazières, A., Fricker, D., Lubetzki, C. & Sol-Foulon, N. Mechanisms of sodium channel clustering and its influence on axonal impulse conduction. Cell. Mol. Life Sci. 73, 723–735 (2016).
Snaidero, N. & Simons, M. The logistics of myelin biogenesis in the central nervous system. Glia 65, 1021–1031 (2017).
Monje, M. Myelin plasticity and nervous system function. Annu. Rev. Neurosci. 41, 61–76 (2018).
Seidl, A. H., Rubel, E. W. & Harris, D. M. Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J. Neurosci. 30, 70–80 (2010).
Waxman, S. G., Black, J. A., Kocsis, J. D. & Ritchie, J. M. Low density of sodium channels supports action potential conduction in axons of neonatal rat optic nerve. Proc. Natl Acad. Sci. USA 86, 1406–1410 (1989).
Johnston, W. L., Dyer, J. R., Castellucci, V. F. & Dunn, R. J. Clustered voltage-gated Na+ channels in Aplysia axons. J. Neurosci. 16, 1730–1739 (1996).
Zeng, S. & Jung, P. Simulation analysis of intermodal sodium channel function. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 78, 061916 (2008).
Neishabouri, A. & Faisal, A. A. Saltatory conduction in unmyelinated axons: clustering of Na+ channels on lipid rafts enables micro-saltatory conduction in C-fibers. Front. Neuroanat. 8, 109–109 (2014).
Rama, S., Zbili, M. & Debanne, D. Signal propagation along the axon. Curr. Opin. Neurobiol. 51, 37–44 (2018).
Chiu, S. Y. & Ritchie, J. M. On the physiological role of internodal potassium channels and the security of conduction in myelinated nerve fibres. Proc. R. Soc. Lond. B Biol. Sci. 220, 415–422 (1984).
Vabnick, I. et al. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J. Neurosci. 19, 747–758 (1999).
Brohawn, S. G., Su, Z. & MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl Acad. Sci. USA 111, 3614–3619 (2014).
Renigunta, V., Schlichthörl, G. & Daut, J. Much more than a leak: structure and function of K2p-channels. Pflug. Arch. 467, 867–894 (2015).
Holland, L., de Regt, H. W. & Drukarch, B. Thinking about the nerve impulse: the prospects for the development of a comprehensive account of nerve impulse propagation. Front. Cell. Neurosci. 13, 208–208 (2019).
Gründemann, J. & Clark, B. A. Calcium-activated potassium channels at nodes of Ranvier secure axonal spike propagation. Cell Rep. 12, 1715–1722 (2015).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014).
Stedehouder, J. et al. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb. Cortex 27, 5001–5013 (2017).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Craner, M. J., Lo, A. C., Black, J. A. & Waxman, S. G. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 126, 1552–1561 (2003).
Craner, M. J., Hains, B. C., Lo, A. C., Black, J. A. & Waxman, S. G. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127, 294–303 (2004).
Dupree, J. L. et al. Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol. 1, 179–192 (2004).
Pfeiffer, F., Frommer-Kaestle, G. & Fallier-Becker, P. Structural adaption of axons during de- and remyelination in the Cuprizone mouse model. Brain Pathol. 29, 675–692 (2019).
Mathis, C., Denisenko-Nehrbass, N., Girault, J. A. & Borrelli, E. Essential role of oligodendrocytes in the formation and maintenance of central nervous system nodal regions. Development 128, 4881–4890 (2001).
Rasband, M. N., Kagawa, T., Park, E. W., Ikenaka, K. & Trimmer, J. S. Dysregulation of axonal sodium channel isoforms after adult-onset chronic demyelination. J. Neurosci. Res. 73, 465–470 (2003).
Black, J. A. et al. Sensory neuron-specific sodium channel SNS is abnormally expressed in the brains of mice with experimental allergic encephalomyelitis and humans with multiple sclerosis. Proc. Natl Acad. Sci. USA 97, 11598–11602 (2000).
Roostaei, T. et al. Channelopathy-related SCN10A gene variants predict cerebellar dysfunction in multiple sclerosis. Neurology 86, 410–417 (2016).
Jukkola, P. I., Lovett-Racke, A. E., Zamvil, S. S. & Gu, C. K+ channel alterations in the progression of experimental autoimmune encephalomyelitis. Neurobiol. Dis. 47, 280–293 (2012).
Howell, O. W. et al. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J. Neuropathol. Exp. Neurol. 69, 1017–1033 (2010).
Bagchi, B. et al. Disruption of myelin leads to ectopic expression of KV1.1 channels with abnormal conductivity of optic nerve axons in a cuprizone-induced model of demyelination. PLoS ONE 9, e87736 (2014).
Hamada, M. S. & Kole, M. H. P. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J. Neurosci. 35, 7272–7286 (2015).
Craner, M. J. et al. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2 + exchanger. Proc. Natl Acad. Sci. USA 101, 8168–8173 (2004).
Coman, I. et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129, 3186–3195 (2006).
Han, C., Huang, J. & Waxman, S. G. Sodium channel Nav1.8: emerging links to human disease. Neurology 86, 473–483 (2016).
Zoupi, L., Markoullis, K., Kleopa, K. A. & Karagogeos, D. Alterations of juxtaparanodal domains in two rodent models of CNS demyelination. Glia 61, 1236–1249 (2013).
Fu, Y. et al. Paranodal myelin retraction in relapsing experimental autoimmune encephalomyelitis visualized by coherent anti-Stokes Raman scattering microscopy. J. Biomed. Opt. 16, 106006 (2011).
Stojic, A., Bojcevski, J., Williams, S. K., Diem, R. & Fairless, R. Early nodal and paranodal disruption in autoimmune optic neuritis. J. Neuropathol. Exp. Neurol. 77, 361–373 (2018).
Crawford, D. K., Mangiardi, M., Xia, X., López-Valdés, H. E. & Tiwari-Woodruff, S. K. Functional recovery of callosal axons following demyelination: a critical window. Neuroscience 164, 1407–1421 (2009).
Wolswijk, G. & Balesar, R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain 126, 1638–1649 (2003).
McDonald, W. I. & Sears, T. A. Focal experimental demyelination in the central nervous system. Brain 93, 575–582 (1970).
Bostock, H. & Sears, T. A. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J. Physiol. 280, 273–301 (1978).
Felts, P. A., Baker, T. A. & Smith, K. J. Conduction in segmentally demyelinated mammalian central axons. J. Neurosci. 17, 7267–7277 (1997).
Shrager, P. Axonal coding of action potentials in demyelinated nerve fibers. Brain Res. 619, 278–290 (1993).
Hamada, M. S., Popovic, M. A. & Kole, M. H. P. Loss of saltation and presynaptic action potential failure in demyelinated axons. Front. Cell. Neurosci. 11, 45 (2017).
Alrashdi, B. et al. Nav1.6 promotes inflammation and neuronal degeneration in a mouse model of multiple sclerosis. J. Neuroinflammation 16, 215 (2019).
Schattling, B. et al. Activity of NaV1.2 promotes neurodegeneration in an animal model of multiple sclerosis. JCI Insight 1, e89810 (2016).
Kapoor, R. et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 9, 681–688 (2010).
Raftopoulos, R. et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 15, 259–269 (2016).
Brugarolas, P. et al. Development of a PET radioligand for potassium channels to image CNS demyelination. Sci. Rep. 8, 607 (2018).
Nikic, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Witte, M. E. et al. Calcium influx through plasma-membrane nanoruptures drives axon degeneration in a model of multiple sclerosis. Neuron 101, 615–624.e5 (2019).
Mathey, E. K. et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med. 204, 2363–2372 (2007).
Stich, O. et al. Prevalence of neurofascin-155 antibodies in patients with multiple sclerosis. J. Neurol. Sci. 364, 29–32 (2016).
Devaux, J. J., Odaka, M. & Yuki, N. Nodal proteins are target antigens in Guillain–Barré syndrome. J. Peripher. Nerv. Syst. 17, 62–71 (2012).
Elliott, C. et al. Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain 135, 1819–1833 (2012).
Kawamura, N. et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology 81, 714–722 (2013).
Derfuss, T. et al. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl Acad. Sci. USA 106, 8302–8307 (2009).
Cortese, A. et al. Antibodies to neurofascin, contactin-1, and contactin-associated protein 1 in CIDP: clinical relevance of IgG isotype. Neurol. Neuroimmunol. Neuroinflamm. 7, e639 (2019).
Delmont, E. et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain 140, 1851–1858 (2017).
Vallat, J.-M. et al. Subacute nodopathy with conduction blocks and anti-neurofascin 140/186 antibodies: an ultrastructural study. Brain 141, e56 (2018).
Querol, L. et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann. Neurol. 73, 370–380 (2013).
Cortese, A. et al. Antibodies to neurofascin, contactin-1, and contactin-associated protein 1 in CIDP: clinical relevance of IgG isotype. Neurol. Neuroimmunol. Neuroinflamm. 7, e639 (2020).
Mathey, E. K. et al. Autoantibody responses to nodal and paranodal antigens in chronic inflammatory neuropathies. J. Neuroimmunol. 309, 41–46 (2017).
Devaux, J. J. et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 86, 800–807 (2016).
Kouton, L. et al. Electrophysiological features of chronic inflammatory demyelinating polyradiculoneuropathy associated with IgG4 antibodies targeting neurofascin 155 or contactin 1 glycoproteins. Clin. Neurophysiol. 131, 921–927 (2020).
Ogata, H. et al. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann. Clin. Transl. Neurol. 2, 960–971 (2015).
Cortese, A. et al. Neurofascin-155 as a putative antigen in combined central and peripheral demyelination. Neurol. Neuroimmunol. Neuroinflamm. 3, e238 (2016).
Sasaki, M. et al. Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord. J. Neurosci. 26, 1803–1812 (2006).
Smith, K. J., Bostock, H. & Hall, S. M. Saltatory conduction precedes remyelination in axons demyelinated with lysophosphatidyl choline. J. Neurol. Sci. 54, 13–31 (1982).
Auer, F., Vagionitis, S. & Czopka, T. Evidence for myelin sheath remodeling in the CNS revealed by in vivo imaging. Curr. Biol. 28, 549–559.e3 (2018).
Reimer, M. M. et al. Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion. J. Neurosci. 31, 18185–18194 (2011).
Ritter, J. et al. Neonatal hyperoxia exposure disrupts axon-oligodendrocyte integrity in the subcortical white matter. J. Neurosci. 33, 8990–9002 (2013).
Marin, M. A. et al. Reassembly of excitable domains after CNS axon regeneration. J. Neurosci. 36, 9148–9160 (2016).
Marion, C. M., Radomski, K. L., Cramer, N. P., Galdzicki, Z. & Armstrong, R. C. Experimental traumatic brain injury identifies distinct early and late phase axonal conduction deficits of white matter pathophysiology, and reveals intervening recovery. J. Neurosci. 38, 8723–8736 (2018).
Eijkelkamp, N. et al. Neurological perspectives on voltage-gated sodium channels. Brain 135, 2585–2612 (2012).
Cooper, E. C. In Jasper’s Basic Mechanisms of the Epilepsies (eds Noebels, J. L. et al.) Chapter 5 (National Center for Biotechnology Information, 2012).
Bauer, C. K. et al. Mutations in KCNK4 that affect gating cause a recognizable neurodevelopmental syndrome. Am. J. Hum. Genet. 103, 621–630 (2018).
Desmazières, A., Sol-Foulon, N. & Lubetzki, C. Changes at the nodal and perinodal axonal domains: a basis for multiple sclerosis pathology? Mult. Schler. J. https://doi.org/10.1177/1352458511434370 (2012).
Acknowledgements
The authors thank the ARSEP foundation, FRM (Fondation pour la Recherche Médicale), Bouvet-Labruyere Family, INSERM-DHOS, APHP (Assistance Publique des Hôpitaux de Paris), “Investissements d’avenir” ANR and NRJ foundation for their precious support related to basic and clinical research on remyelination. We thank Jean Michel Vallat for the electron micrograph of the node of Ranvier, and Rémi Ronzano, Thomas Roux and Elisa Mazuir for assistance with the figures. The authors thank Bernard Zalc for fruitful discussions and careful reading of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, discussions of the content, writing the article, and review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks C. Faivre-Sarrailh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lubetzki, C., Sol-Foulon, N. & Desmazières, A. Nodes of Ranvier during development and repair in the CNS. Nat Rev Neurol 16, 426–439 (2020). https://doi.org/10.1038/s41582-020-0375-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-0375-x
This article is cited by
-
Reduced length of nodes of Ranvier and altered proteoglycan immunoreactivity in prefrontal white matter in major depressive disorder and chronically stressed rats
Scientific Reports (2023)
-
Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles
Nature Reviews Neuroscience (2023)
-
Node of Ranvier remodeling in chronic psychosocial stress and anxiety
Neuropsychopharmacology (2023)
-
Revealing nanostructures in brain tissue via protein decrowding by iterative expansion microscopy
Nature Biomedical Engineering (2022)
-
Long-term in vivo imaging of mouse spinal cord through an optically cleared intervertebral window
Nature Communications (2022)